Multiparametric MRI and radiogenomics in prostate cancer
Prostate cancer is a prevalent malignancy that continues to pose significant challenges in diagnosis, risk stratification, and treatment planning. In recent years, there has been a paradigm shift in medical imaging towards a more comprehensive and quantitative approach. Multiparametric Magnetic Resonance Imaging (mpMRI) has emerged as a powerful tool for prostate cancer assessment, providing detailed anatomical and functional information. Radiomics, a cutting-edge field in medical imaging, extends beyond traditional image interpretation by extracting and analyzing a vast array of quantitative features from medical images.
DCE-MRI radiomics for prostate cancer detection
Multiparametric MRI, including T2-weighted, diffusion weighted and Dynamic Contrast-Enhanced (DCE)-MRI, is the recommended imaging modality in the standard prostate cancer diagnostic workflow. Recent imaging guidelines (PI-RADS v2) downgraded the value DCE-MRI in the diagnosis of PCa. However, a purely qualitative analysis of the DCE-MRI time series, as it is generally done by radiologists, might overlook information on the microvascular architecture and function.
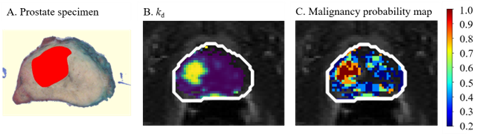
Motivated by this, we investigated whether a radiomic combination of spatial and temporal features extracted from DCE-MRI improved the detection of prostate cancer. Our results show that a selected combination of quantitative spatial and temporal features extracted from DCE-MRI and incorporated in machine learning classifiers obtains a good diagnostic performance (AUC = 0.80-0.86) in distinguishing malignant from benign regions.
Deep-learning for prostate zonal segmentation
Prostate zonal segmentation is a pivotal step in the automation of prostate cancer (PCa) diagnosis, MRI-guided radiotherapy, and focal treatment planning. In this study, we introduce a multi-channel U-Net for automatic prostate zonal segmentation, capable of accommodating multiple MRI sequences.
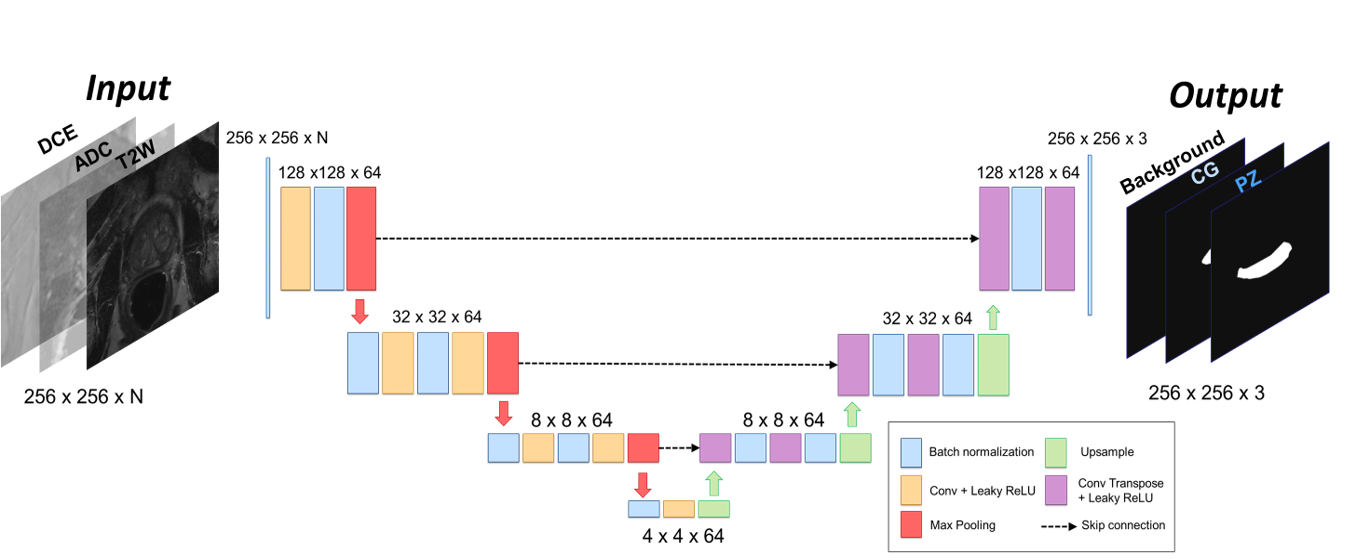
Leveraging a multicenter, multiparametric MRI dataset, we examine the model’s robustness in the face of varied acquisition protocols and assess whether the inclusion of additional imaging sequences enhances segmentation performance. Our findings reveal that T2-weighted imaging alone proves sufficient for successful prostate zonal segmentation. Notably, despite utilizing a modest multicenter dataset, the models exhibit robustness across acquisition protocols, yielding performance comparable to that achieved with larger datasets sourced from a single institute. This research contributes valuable insights towards streamlining and optimizing automated prostate zonal segmentation, underscoring the potential of this methodology in diverse clinical settings.
Radiogenomics of prostate cancer
Radiogenomics explores the relationship between imaging features and underlying genomic alterations within the tumor. This synergy between imaging and genomics provides a holistic view of the disease, offering insights into the molecular landscape that traditional imaging alone may not capture. This may not only enhances diagnostic accuracy and risk stratification but also paves the way for personalized treatment strategies.
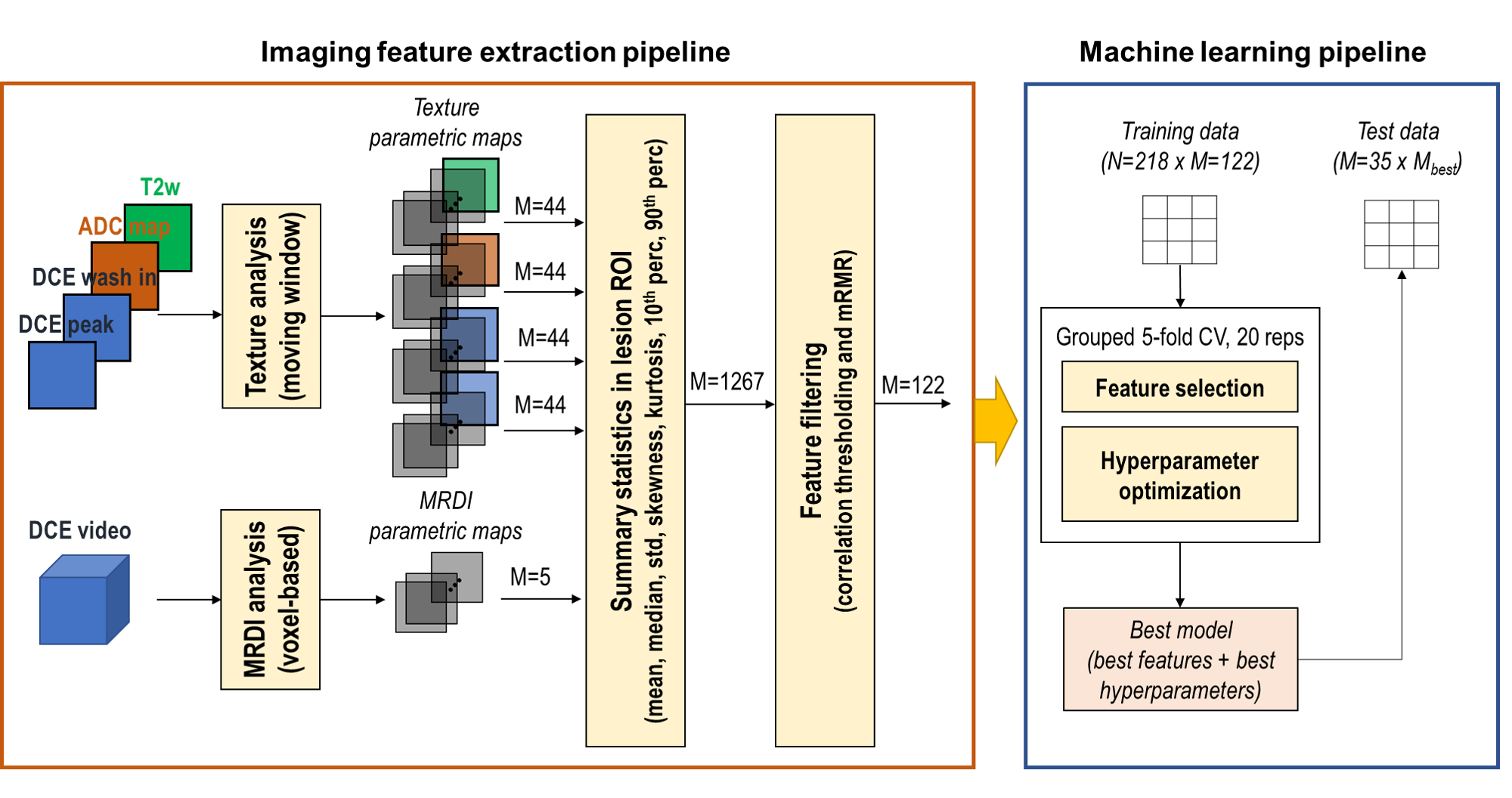
Leveraging a unique datasets, including prostate cancer patients who underwent mpMRI and whose biopsied tumor tissue where sequenced, we explored radiogenomics in prostate cancer. We investigated whether imaging features extracted from multiparametric MRI correlated with transcriptomics features extracted from RNAseq. To this end, we developed machine learning frameworks to identify the most relevant imaging and transcriptomics features and analyzed the correlation between them.
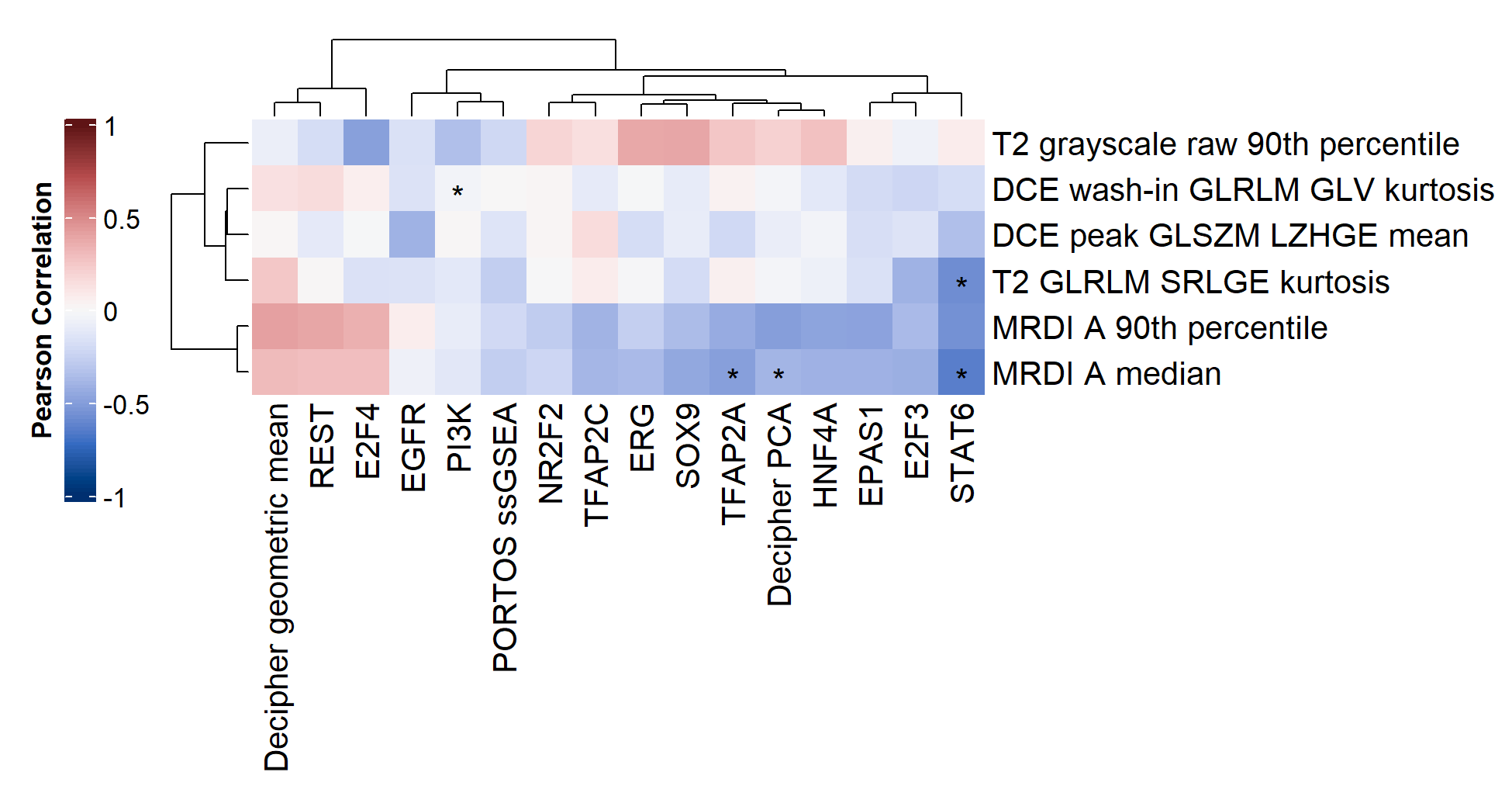
We found a significant link especially between perfusion-based imaging features, extracted from MRDI, with transcriptomics features related to related to cell proliferation and migration, and associated with prostate cancer aggressiveness and progression.
Contributors and partners
BM/d lab:
- Catarina Dinis Fernandes (assistant professor)
- Hubert Blach (former master student)
- Annekoos Schaap (former master student)
Partners:
- Biomodelling group (ICMS, Biomedical Engineering), TU/e
- Amsterdam University Medical Center
- Netherlands Cancer Institute (Antoni van Leeuwenhoek)
- Radboud University Medical Center
Funding:
- Hanarth Fonds fellowship
- KNAW Van Leersum grant

