Quantitative contrast-enhanced ultrasound
Contrast-enhanced ultrasound (CEUS) has revolutionized medical imaging by introducing a dynamic dimension to conventional ultrasound techniques. With the use of ultrasound contrast agents (UCAs), such as microbubbles, CEUS enables enhanced visualization of blood flow and tissue perfusion. Beyond mere qualitative assessments, the field of CEUS has increasingly focused on quantification – a pivotal aspect that provides precise and measurable insights into vascular and tissue characteristics. Quantitative analysis in CEUS involves the application of sophisticated methodologies, including temporal, spatial, and spatiotemporal analyses of contrast agent kinetics. This approach not only refines diagnostic accuracy but also plays a crucial role in deciphering pathological conditions. In this line of research, we develop quantitative analysis methods to extract multiple quantitative maps from CEUS, reflecting complementary variables of underlying physiological processes. Using probabilistic frameworks based on emerging machine-learning methods, we aim to enhance the diagnostic accuracy of CEUS imaging through the optimal combination of extracted complementary information.
A general introduction on this topic can be found on our review paper “Contrast-Enhanced Ultrasound Quantification: From Kinetic Modeling to Machine Learning.”.
CEUS for hemodynamic quantification
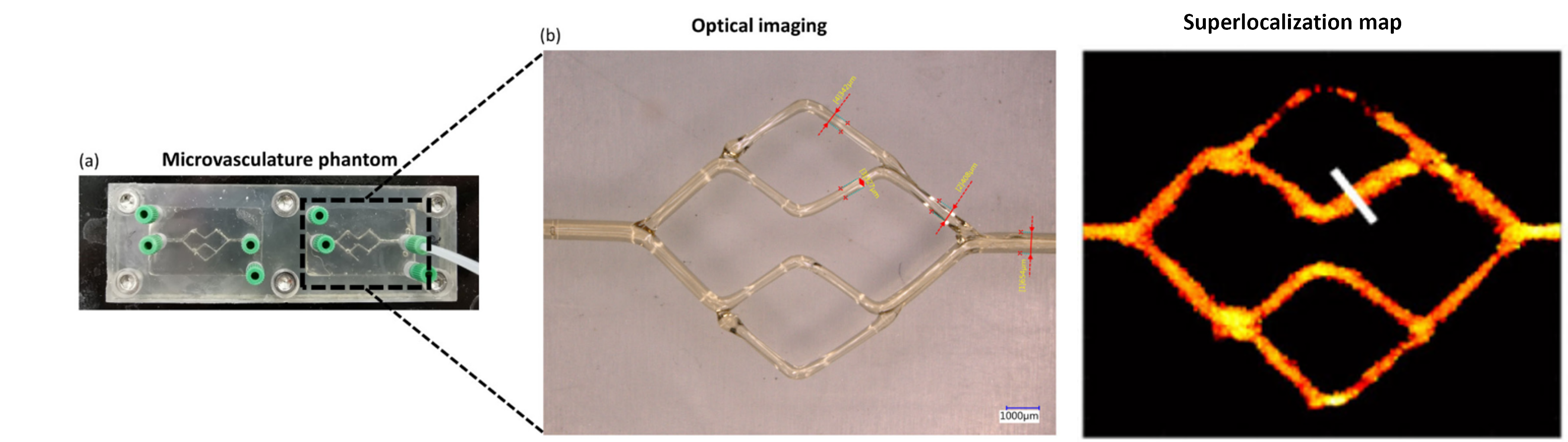
Over the past decades, significant advancements in hemodynamic quantification methods have evolved based on CEUS imaging. In particular, our group has developed contrast-ultrasound dispersion imaging (CUDI) techniques, allowing the quantification of cancer angiogenesis. These techniques involve temporal, spatial, and spatiotemporal analysis of CEUS acquisitions to extract parameters related to blood perfusion and contrast dispersion. Encouragingly, CUDI techniques have demonstrated promising results in localizing prostate cancer. Despite these advancements, the clinical translation of many methods faces challenges such as the lack of validation under controllable conditions, limited understanding of the relationship between hemodynamics and tissue properties, and issues with imaging efficiency, reliability, and diagnostic accuracy. Therefore, the primary goal of this project was to facilitate the clinical translation and enhance the clinical value of a set of CEUS-based hemodynamic quantification techniques. With this goal in mind, we initially design and construct controllable, realistic, and perfusable (micro)vascular phantom models. These models are utilized for conducting quantitative hemodynamics analysis and optimizing contrast-enhanced ultrasound (CEUS) imaging efficiency in vitro. Subsequently, we embark on the development and validation of hemodynamic quantification methods specifically tailored for implementation in existing clinical scanners. The emphasis is on enhancing their diagnostic accuracy in vivo. This line of research is summarized in the work of thesis of Peiran Chen.
Quantitative CEUS for classification of liver lesion
Liver cancer is among the most common cancers globally. In 2020, it was estimated to be the sixth most diagnosed cancer and the third leading cause of cancer-related deaths worldwide. Imaging plays a crucial role in the diagnosis, staging, and monitoring of liver cancer. However, due to the limitations of current diagnostic tools, focal liver lesions (FLLs) are often diagnosed incidentally, when the cancer is already in an advanced stage. Therefore, early differentiation between benign and malignant FLLs is vital to ensure the timely initiation of appropriate treatment.
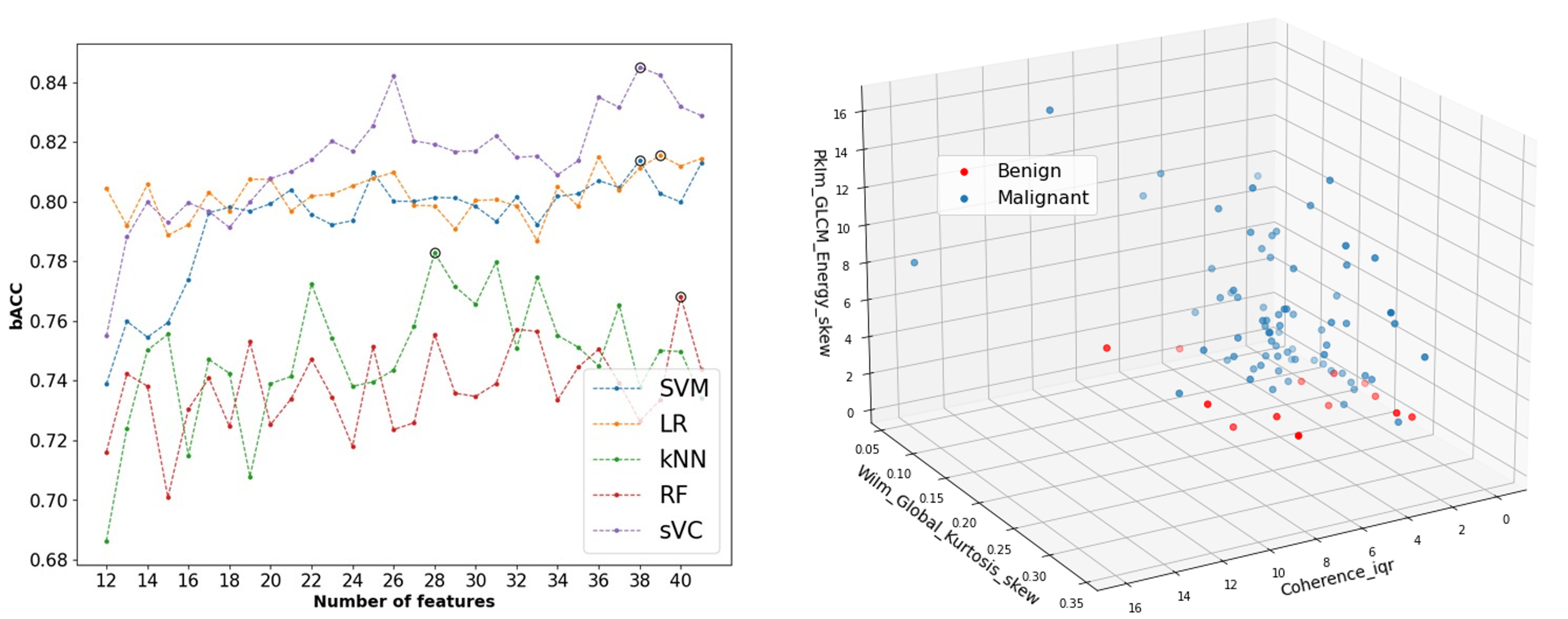
Motivated by this, we proposed an interpretable radiomics method to distinguish between cancerous and non-cancerous focal liver lesions (FLLs) using contrast-enhanced ultrasound (CEUS). While CEUS holds promise for diagnosing FLLs, current methods rely solely on qualitative analysis, often hindered by motion artifacts and the complex nature of liver contrast enhancement. To overcome these challenges, we propose combining temporal and spatiotemporal analysis of arterial phase enhancement by CUDI, with texture analysis at various time points. Machine learning classifiers are then trained using these features for semi-automated FLL characterization. Clinical validation on 87 FLLs from 72 at-risk patients showed promising results, with a balanced accuracy of 0.84 in distinguishing between benign and malignant lesions. Feature analysis highlights the importance of combining spatiotemporal and texture features for optimal performance, particularly focusing on microvascular perfusion, architecture, and spatial enhancement characteristics.
Ultrasound imaging of the liver is, however, unavoidably affected by motion. This limits the robustness of feature extraction and hinders the application of additional quantification techniques which are affected by motion. To overcome this challenge, we proposed a motion compensation algorithm, named the Iterative Local Search Algorithm (ILSA). This algorithm effectively addresses both periodic and non-periodic in-plane motion while eliminating frames with out-of-plane motion in contrast-enhanced ultrasound (CEUS) cines. We tested the algorithm on 183 focal liver lesions in 155 patients from three hospitals. Quantitatively, we observed a significant decrease in median root mean square error and in B-mode intensity standard deviation, and a significant increase in R2. Qualitatively, B-Mode mean intensity projection images indicated improved spatial resolution, and parametric perfusion imaging demonstrated enhanced spatial detail and better differentiation between lesions and background liver parenchyma. ILSA proves effective in compensating for various motion types encountered during liver CEUS, potentially enhancing the quantification of contrast signals in FLLs.
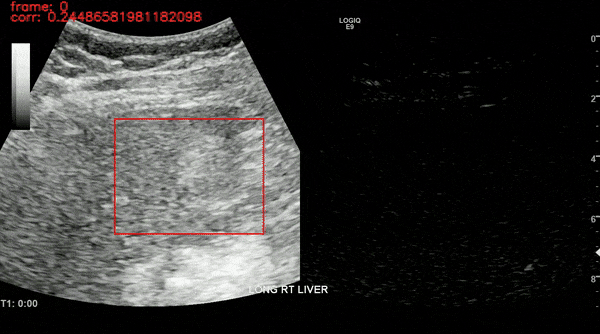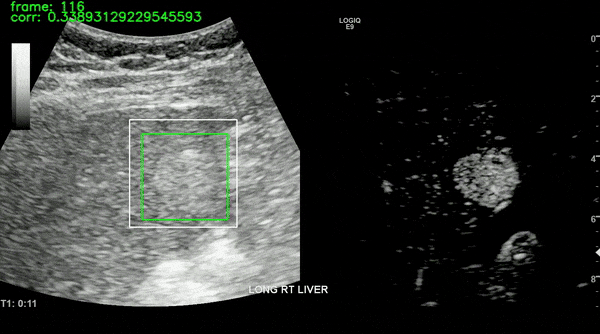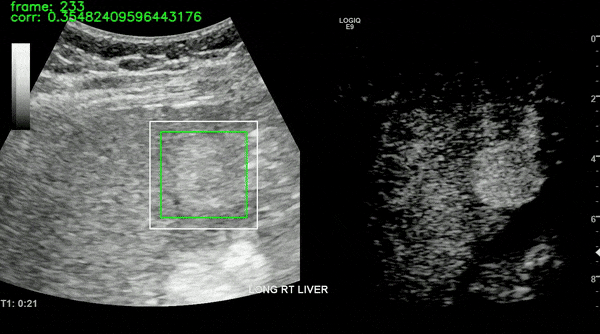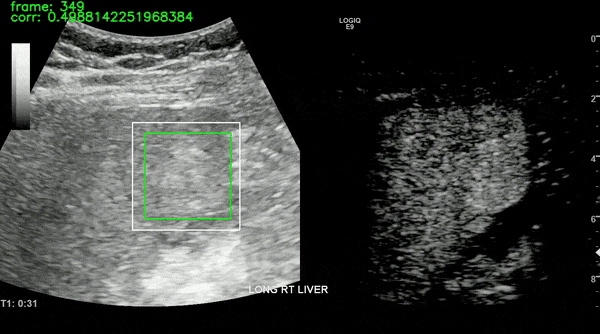
All the work on CEUS for liver cancer imaging has been supported by Dr. Ahmed El Kaffas at Stanford Medicine, in collaboration with Thomas Jefferson University Hospital (Dr. Andrej Lyshchik and Dr. John R. Eisenbrey). The ILSA method was developed in collaboration with Thodsawit Tiyarattanachai at Stanford Medicine.
Quantitative CEUS for breast lesions differentiation
Breast cancer remains a paramount global health concern, affecting millions of lives and necessitating comprehensive efforts in understanding, diagnosing, and treating the disease. According to recent statistics, breast cancer is the most common cancer among women worldwide, with approximately 2.3 million new cases diagnosed annually and over 685,000 reported deaths. Beyond its prevalence, the impact of breast cancer extends beyond mere numbers, affecting individuals physically, emotionally, and socioeconomically. Imaging by mammography and B-mode ultrasound, clinical breast examinations, and self-breast examinations have traditionally been pivotal in the early diagnosis of breast cancer. However, these diagnostic strategies come with their limitations, including false positives and negatives, discomfort, and exposure to ionizing radiation in the case of mammography. In screening protocols, US primarily complements mammography and proves more advantageous for individuals with dense breast tissue. When identifying a potentially concerning lump or mass, US plays a vital role in elucidating the characteristics of the abnormality. However, the assessment of morphological attributes through B-mode images often results in a considerable number of false-positives, prompting unnecessary biopsy procedures. In this project, we investigated whether CEUS could improve the differentiation of breast lesions. We employed spatiotemporal analysis of CEUS to quantify heterogeneity by parameters such as spectral coherence, temporal correlation and mutual information, which are related to the contrast agent dispersion through the vascular network. In a poplutaion of 64 women with well-perfused lesions we achieved a AUC of 0.89 for differentiating between benign and malignant breast lesions. This is the first time that CUDI has been applied on the breast, with very encouraging results!
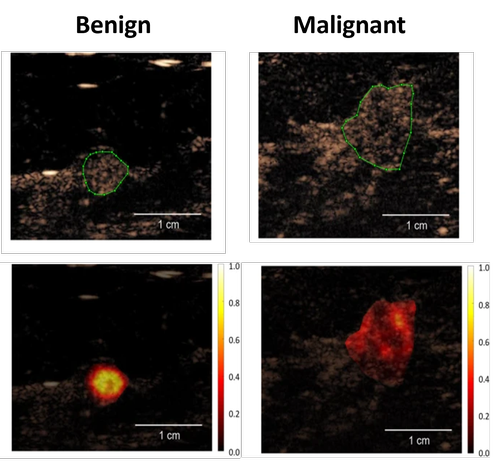
Contributors and partners
BM/d lab:
- Peiran Chen (former PhD student)
- Chuan Chen (former Postdoc researcher)
- Hessel Wijkstra (part-time professor)
- Massimo Mischi (full professor, head of BM/d)
Collaborators:
- Microsystems group, TU/e
- Mixed Signal Microelectronics, TU/e
- Solstice Pharmaceuticals
- Bracco S.p.A
- Philips Research
- Amsterdam University Medical Center
- Stanford Medicine
- Thomas Jefferson University Hospital
Funding:
- Hanarth Fonds fellowship
- Knaw Van Leersum grant
- NWO OTP, LOCATE project
- ERC Proof-of-Concept
- 4TU High Tech for a sustainable future, Precision Medicine program

