Nanobubble-based contrast-enhanced ultrasound
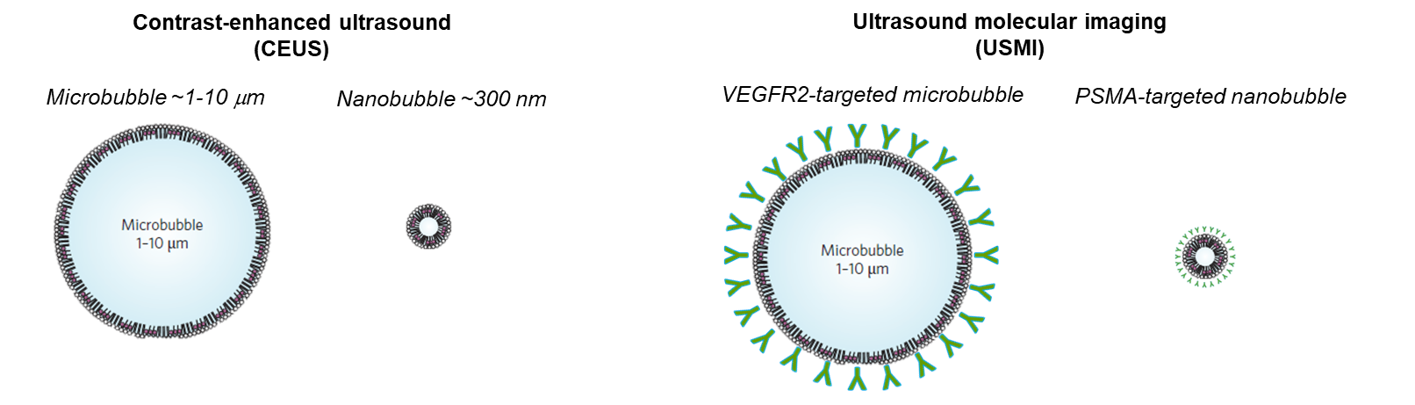
Traditional contrast agents have played a pivotal role in improving the visibility of anatomical structures and abnormalities in medical imaging. However, the quest for agents with enhanced imaging capabilities, reduced side effects, and increased target specificity has driven the exploration of nanobubbles. This miniature gas-filled structures with typical size ten times smaller than microbubbles penetrate more easily in the capillary network, and may extravasate in case of enhanced permeability such as in cancer and inflammation. When their shell is decorated with suitable ligands, they can then reach targets that go beyond the vessel wall. Because of their more complex kinetics and distribution in and out of the vasculature, dedicated models and quantification strategies are needed - this is the focus of this line of research.
The second wave phenomenon
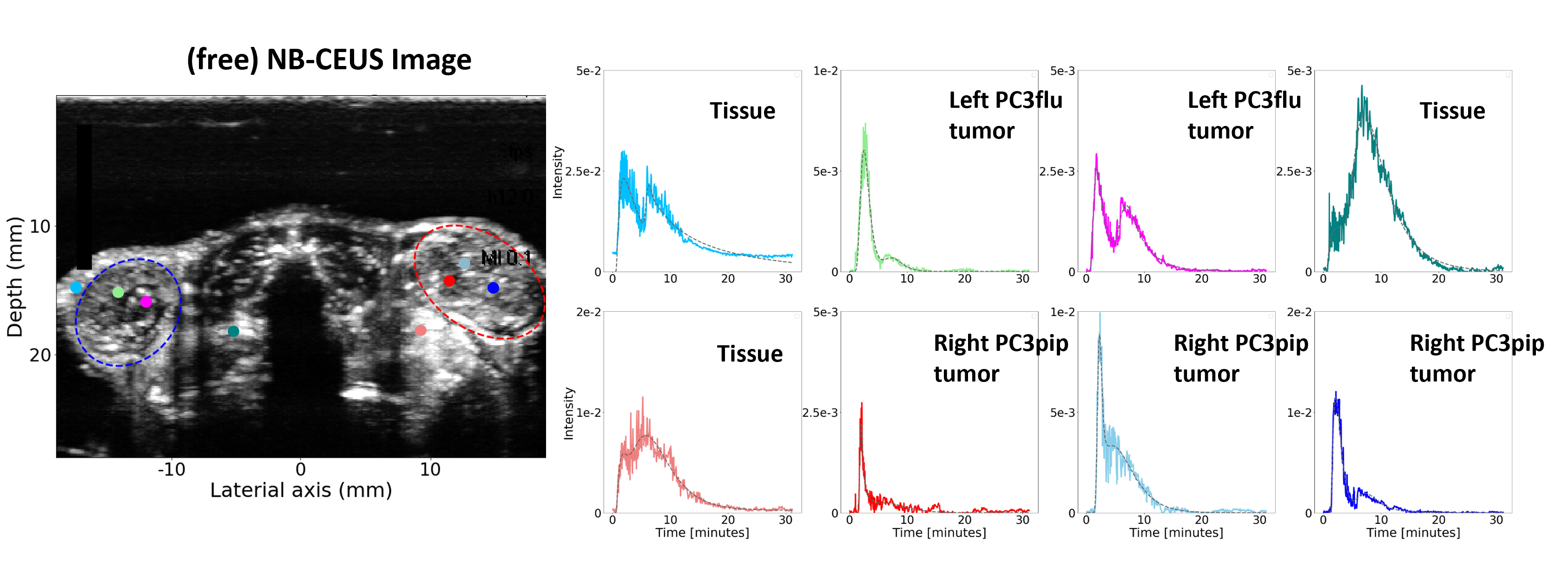
The investigation into nanobubble (NB) pharmacokinetics in contrast-enhanced ultrasound (CEUS) at the pixel level has unveiled a distinctive phenomenon by which the initial passage of the contrast agent bolus is accompanied by a second wave. Notably, this effect has not been previously observed in CEUS utilizing microbubbles. This motivated us to explore and understand this second-wave phenomenon and its potential clinical applications. We characterized this phenomenon by a dedicated pharmacokinetic model and used this model to fit time-intensity curves (TICs) extracted from NB-CEUS. The resulting model parameters were presented as parametric maps to visually depict the characteristics of tumor lesions. Two model parameters, namely the ratio of peak intensities of the second wave over the first and the decay rate of the wash-out process, exhibited significant differences between malignant tumors and normal tissue, suggesting that this unique phenomenon has the potential to support the diagnosis of cancerous lesions.
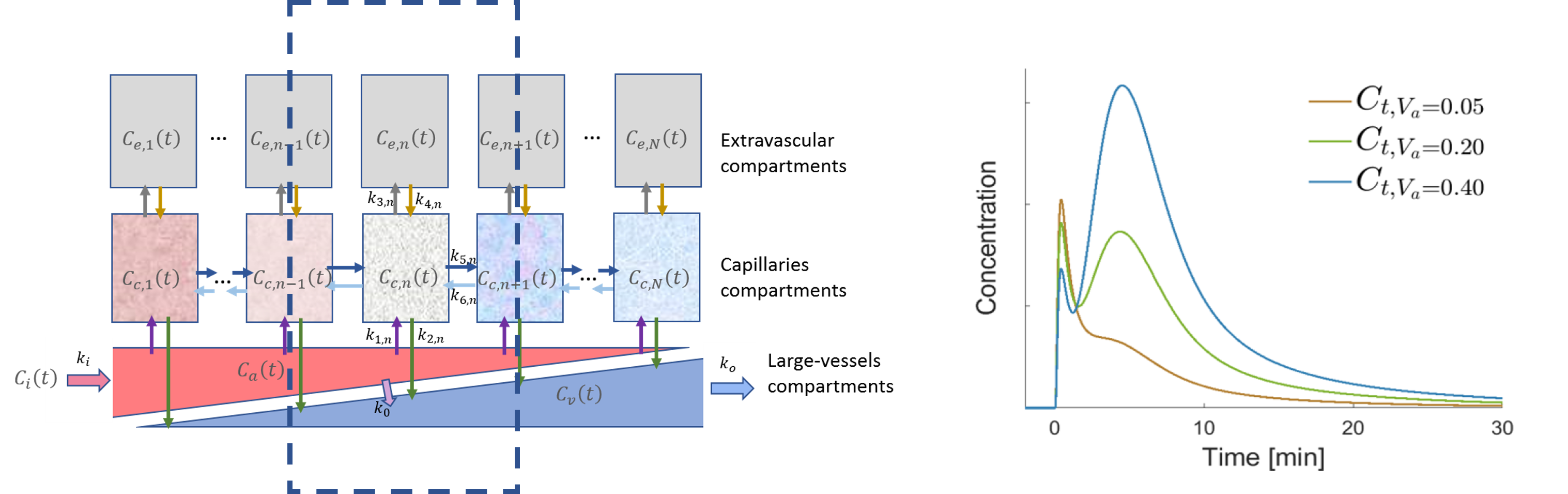
Motivated by the promising findings, we developed novel compartmental pharmacokinetic models designed to elucidate the second-wave phenomenon. Utilizing this models, a more detailed analysis becomes possible, allowing for an in-depth examination of the influence of various physiological factors on the attributes of the second-wave phenomenon. We found that a double modified local density random walk (mLDRW) model, despite its simplicity and reliance on robust assumptions, enables precise fitting of the experimental data, facilitating the estimation of multiple parametric maps for the quantitative and visual representation of in-vivo pharmacokinetics of NBs. Our findings confirm that the second-wave phenomenon is influenced by various physiological factors, highlighting the model’s potential for application in pertinent clinical scenarios.
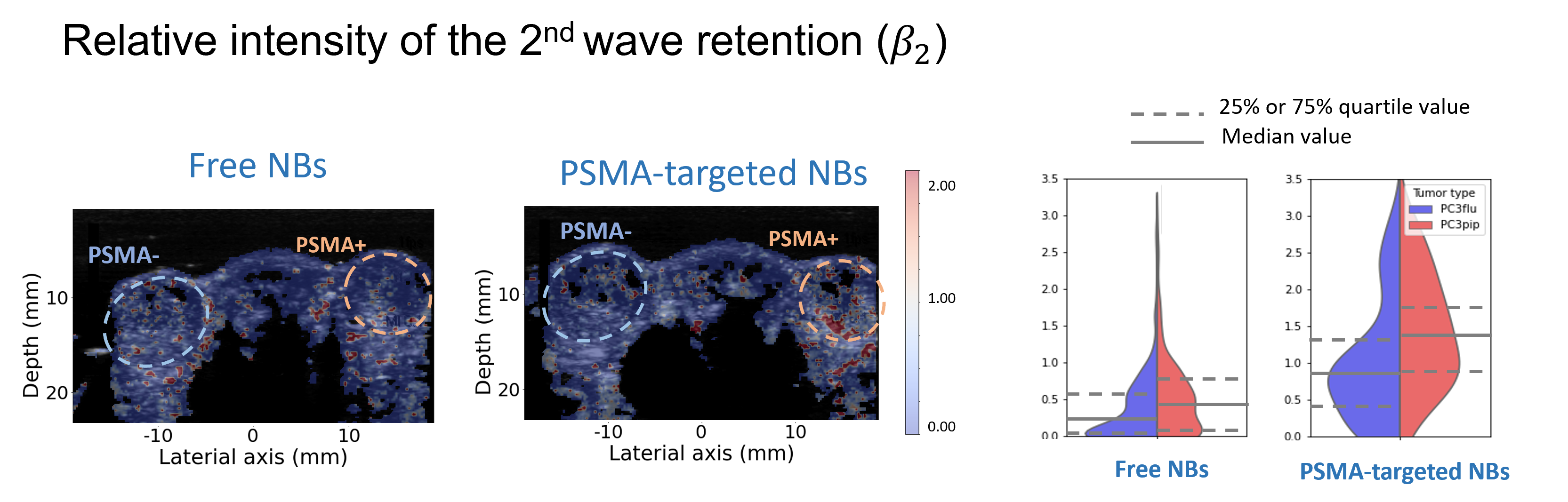
PSMA-targeted nanobubbles
Besides being able to cross the vessel wall, nanobubbles can also be decorated with ligands on their shell to target specific molecules. One relevant target for prostate cancer is the prostate-specific membrane antigen (PSMA), over-expressed on prostate-cancer cells. It has been established that PSMA-targeted NBs can effectively attach to the receptors of prostate cancer cells, exhibiting an extended retention effect in dual-tumor mouse models.
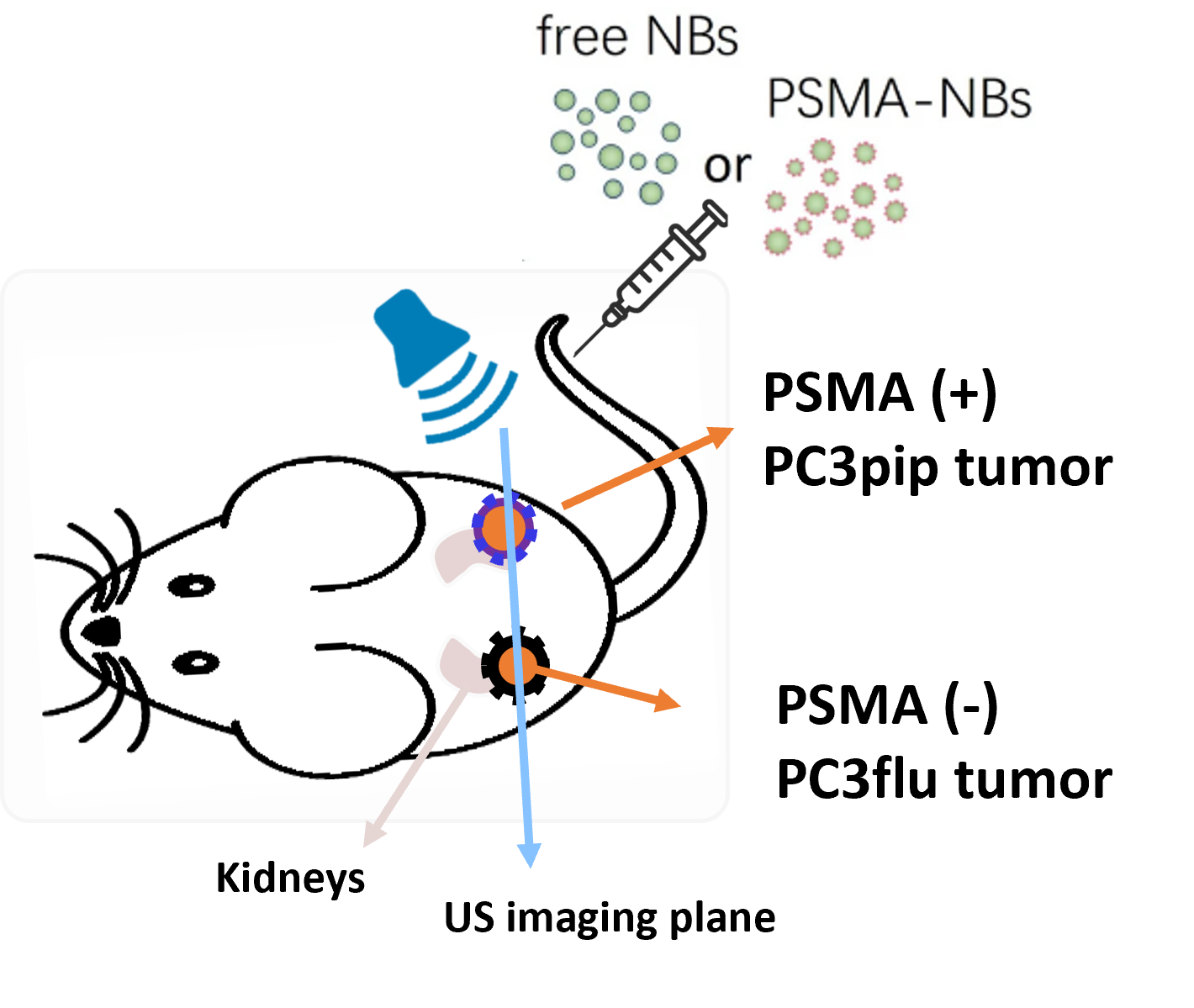
Nevertheless, the assessment of this prolonged retention effect has been constrained to qualitative or semi-quantitative methods thus far. To describe the kinetics of targeted nanobubbles we proposed two pharmacokinetic models, one based on a vascular input given by a mLDRW model, while the other takes inspiration from the reference tissue model, previously developed for nuclear imaging. Our results show that several parameters from these two models are able to distinguish between the kinetics of targeted and free nanobubbles.
Motivated by this, we further set out to investigate whether the estimated parameters were different between cancer and healthy in dual-tumor mouse models of prostate cancer. The dual tumor model is ideal to test our hypothesis because it features one tumor expressing PSMA (PSMA-) on one flank of each mouse, and one tumor not expressing PSMA (PSMA+) on the other flank of the mouse. We found that, compared with the TICs of free NBs, TICs of PSMA-NBs present higher peak intensity and a more delayed second wave, especially in the PSMA+ tumor. This is reflected by two parameters, namely the peak time and residual factor of the second wave, which were significantly different between PSMA+ and PSMA- tumors when using PSMA-NBs.
Contributors and partners
BM/d lab:
- Chuan Chen (former Postdoc researcher)
- Massimo Mischi (full professor, head of BM/d)
Partners:
- Exner lab, Case Western Reserve University
- Kolios lab, Toronto Metropolitan University
Funding:
- 4TU High Tech for a sustainable future, Precision Medicine program

